You might be amazed to hear that scientists are pretty much like ordinary human beings. Well, that’s my view anyway and I can adduce lots of personal quirks if required. But I think the assertion holds generally: we all want to do something that makes a splash and then try hard not to be smug. Scientists fancy ourselves as literati and nowadays biologists can even indulge in cellular art thanks to the wonders of molecular biology. And we love new gizmos — unsurprisingly as they’ve been made by one of our number. And that, of course, makes us fad-prone. Nothing wrong with that, so long as the newest method/bit of kit is used with discretion. Indeed, now more than ever, scientific progress is almost always limited by available methods — and when a new one comes along we love a bandwagon as much as anyone!
Doing our best
The Nobel Prize winner and wonderful science communicator Peter Medawar came to public attention by publishing in 1967 a collection of essays on creativity and originality in science called The Art of the Soluble. Medawar is a personal hero because he gave me my first proper job in science but I wonder if today he might go for The Art of the Possible as a title — albeit risking being accused of nicking it from politics. Seemingly Otto von Bismarck described politics as “the art of the possible, the attainable — the art of the next best.” And science is about doing what you can with what’s available.
All of which is a way of bringing us to a new step in unravelling the mystery of metastasis — how tumour cells escape from a primary growth, spread around the body and found new growths at distant sites. It takes up the story of a recent piece (Where Do Tumours Come From?) but uses a related approach — differential gene expression — which is indeed something of a current bandwagon.
In Where Do Tumours Come From? we saw that it’s possible to engineer mice genetically so that the patterns of gene expression in individual cells can be followed as they develop in a tumour (develop here meaning anything from staying put in the primary tumour to spreading in a highly aggressive manner). These patterns are ‘gene expression signatures’ — much prized in molecular biology these days as they imply which proteins are needed to drive a particular biological effect, in this case tumour cell movement. Though note that a ‘transcriptional signature’ doesn’t mean that any of the genes involved have metastasis-promoting activity.
What’s new and how was it done?
The movers and shakers were Richard Gilbertson and colleagues from the Cancer Research UK Cambridge Institute, University of Cambridge, and from other centres in the UK and the USA. What they did was to compare the transcriptome of normal cells with corresponding tumour cells — a transcriptome being the full range of messenger RNA molecules expressed by an organism (i.e. when you compare normal with tumour you see which genes have gone up in expression and which have gone the other way — inference: the more mRNA the more of the protein it encodes and the converse).
The fun way of displaying such results is in a volcano plot (a type of scatter-plot for large data sets showing significance versus fold-change on the y and x axes, respectively). Fun they may be but the problem is that we all know what they’ll show before we see them: lots of genes go up and lots go down. So where does that leave us? The answer is needing a bit of luck — because it’s really a fishing expedition — and that came the way of Gilbertson & Co. in the form of the gene Nalcn that stood out from the rest (Fig. 1) in that something was already known about what it did. The protein (NALCN) is an ion channel that is responsible for the background leak of sodium ions in central nervous system tissues but it is also made in a range of other tissues and organs.
Fig. 1. NALCN loss-of-function in aggressive cancers. A volcano plot shows differential gene expression between normal gastric cells and gastric adenocarcinomas (downregulated ion channels are highlighted: Nalcn etc). The y axis is the log of the P value (significance); the x axis is the log of the ratio of expression in adenocarcinoma versus normal gastric cells. The left hand half (with green dots: zero to -40) shows genes with reduced expression in the tumours. From Rahrmann et al., 2022.
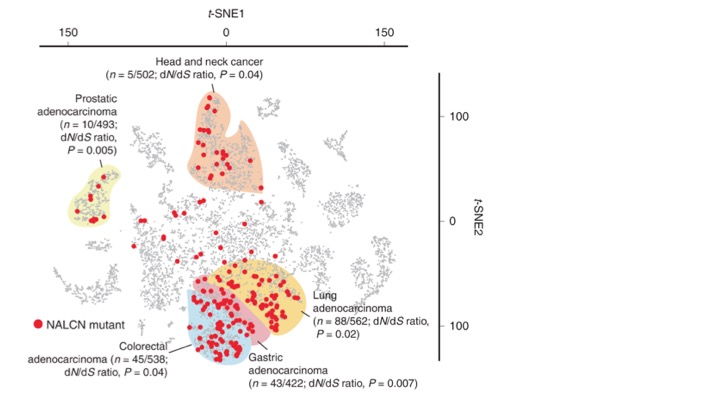
Fig. 2. t-SNE plot of 10,022 human cancers. Non-synonymous mutations in NALCN were enriched in gastric, colorectal, lung, prostate and head and neck cancers, shown by red dots. (The ratio n is the proportion of cancers with mutated NALCN; dN/dS quantifies the strength of selection: it compares synonymous substitution rates (dS) —assumed to be neutral — with non-synonymous substitution rates (dN), which are exposed to selection as they change the amino acid composition of a protein. P = significance). From Rahrmann et al., 2022. For a description of t-SNEs see Peering into our nooks and crannies.
Fig. 2 shows that non-synonymous mutations (ones that change the protein sequence) in NALCN occurred not only in gastric tumours but also in colorectal, lung, prostate and head and neck cancers. Loss of Nalcn in these tumours in mice did not affect tumour incidence but it increased the number of circulating tumour cells (CTCs) and metastases. Treatment of these mice with gadolinium — a NALCN channel blocker— similarly increased CTCs and metastases.
The bottom line
It’s not clear why mutations that close the NALCN channel should have an effect on metastasis though it may be that this effect regulates gene transcription, as has been reported for calcium ion channels. But never mind! Exactly ‘how?’ matters less than the fact that it, in regulating cell shedding from a range of tumour types, NALCN separates the processes of tumour growth and metastasis. It therefore becomes a potential therapy target — drugs capable of reopening the NALCN channel might be anti-metastatic.
More than that, the action of NALCN may be part of the explanation for the puzzling fact that metastases can emerge many years after removal of a primary tumour — given that we know that epithelial cells can carry lots of oncogenic mutations yet behave normally (The Blink of an Eye). Perhaps all they need to kick off is for NACLN function to be lost and away they go!
Reference
Rahrmann, E.P., Shorthouse, D., Jassim, A. et al. The NALCN channel regulates metastasis and nonmalignant cell dissemination. Nat Genet (2022). https://doi.org/10.1038/s41588-022-01182-0.