What better way to celebrate a New Year than with a visit to an art gallery? Well, an alternative might be to scan the cell biology literature but, if you want to cut corners, revisit some of the recent items in this blog.
In Boldly Going we described a method for mapping cell types within tissues on the basis of RNA expression. This identified thousands of RNAs in each cell and, in picking out their spatial pattern, produced images that resemble a pointillist painting — hence the package was christened Seurat by its creators.
That’s very informative but the cellular crock of gold, so to speak, is protein expression — because they do the work in cells — so the next step (Mosaic Masterpieces) revealed how a panel of antibodies could pick out 36 different types of cell in the environment of breast tumours and how the patterns of cells related to patient survival. The tagged cell images also resemble paintings using the pointillism technique developed by Georges Seurat and Paul Signac towards the end of the 19th century, a comparison strengthened by the remarkable comment said to have been made by Seurat that “Great things are done by a series of small things brought together. Some say they see poetry in my paintings; I see only science.”
The next small thing
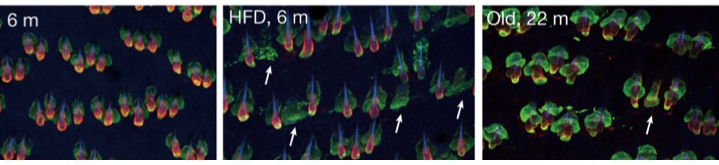
Xiao Qian Wang, Raza Ali and colleagues from the CRUK Cambridge Institute and numerous centres across Europe and extending to Taiwan have given a perfect example of the truism of Seurat’s words in contemporary science. Taking up the methodology of Mosaic Masterpieces, the question they asked was: “Why does immune checkpoint blockade (ICB) work in some patients with triple-negative breast cancer but not in others?” [ICB uses a class of drugs known as immune checkpoint inhibitors {e.g., pembrolizumab (Keytruda) and ipilimumab (Yervoy)}. Much like other immunotherapies, the goal of checkpoint blockade immunotherapy is to strengthen the body’s immune system and enhance its ability to fight off harmful invaders]. Because ICB targets cell-cell interactions, they looked at the type of cells in the tumour microenvironment and how it is changed by ICB (it’s an extension of the work of Keren & Co on triple negative breast cancer described in Mosaic Masterpieces). They tracked 43 different proteins and the montage below gives an artistic impression of their efforts.
Representative images of protein expression in tumour samples. 43 different proteins were detected by imaging mass cytometry (IMC). The image labels refer to proteins detected on specific cells. E.g., CD20 is a general marker for most B-cells, TOX and PD-1 are cytotoxic T cells, TOX and OX40 are T helper cells. PanCK = cytokeratins (keratins).To pursue any of these proteins try The Human Gene Nomenclature database at: https://www.genenames.org/tools/search/.White scale bars = 50 μm. From Wang et al., 2023.
Never mind the art, what about the science?
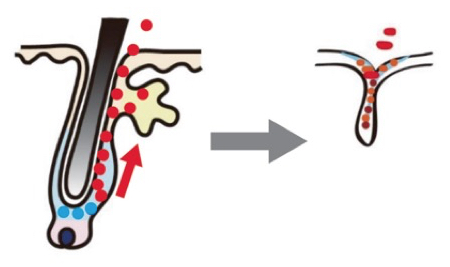
The critical findings were that the levels of a sub-population of T cells (CD8+TCF1+T cells) and of tumour cells that express MHCII+ were dominant predictors of response. In addition, interactions between tumour cells and two other types of immune cells (B cells and granzyme B+ T cells) were important. In other words, ICB treatment remodels the tumour environment and tumours that respond to treatment have lots of granzyme B+ T cells whereas resistant tumours are rich in CD15+ cancer cells. This effect is shown schematically in the image below. [Terminology: CD8 (cluster of differentiation 8): a transmembrane glycoprotein that is a co-receptor for the T-cell receptor (TCR). TCF1 is a transcription factor that is essential for early T cell development. Granzyme B is a caspase-like serine protease that is released by cytotoxic lymphocytes to kill virus-infected and tumour cells. Major histocompatibility complex (MHC) class II molecules present processed antigens, derived primarily from exogenous sources, to CD4(+) T-lymphocytes].
Spatial interactions in a responsive tumour. Here immunotherapy has given rise to a high level of epithelial–CD8+GZMB+T cell interactions. White lines mark tumour epithelial cells interacting with CD8+GZMB+T cells. White scale bar = 50 μm. From Wang et al., 2023.
Taken together these results show that the organization of cell types within the tumour microenvironment determines whether ICB works or not. The key implication is that biopsies taken in the early stages of ICB may give a clear guide as to whether the treatment is working.
As Seurat might have observed, ‘Small steps, small steps’. Very true — but this work tags over 40 different types and sub-types of cell coming and going in the tumour locale. We can be sure that none of them are there as a contribution to art. Thus we need to tease apart all these players in the tumour ecosystem if we are to reach the goal of precision immuno-oncology.
Reference
Wang XQ, Danenberg E, Huang CS, Egle D, Callari M, Bermejo B, Dugo M, Zamagni C, Thill M, Anton A, Zambelli S, Russo S, Ciruelos EM, Greil R, Győrffy B, Semiglazov V, Colleoni M, Kelly CM, Mariani G, Del Mastro L, Biasi O, Seitz RS, Valagussa P, Viale G, Gianni L, Bianchini G, Ali HR. Spatial predictors of immunotherapy response in triple-negative breast cancer. Nature. 2023 Sep;621(7980):868-876. doi: 10.1038/s41586-023-06498-3. Epub 2023 Sep 6. PMID: 37674077; PMCID: PMC10533410.