One of the major problems currently faced by the field of cancer therapy is the difficulty of predicting how an individual will respond to treatment. This is especially true of breast cancer when chemotherapy is used before surgery — called neoadjuvant therapy — with the aim of increasing survival rates after breast-conserving surgery.
As ever, there’s been no shortage of effort. A number of studies have taken molecular and pathology data, looked for anything that might predict overall response and failed to come up with clear indicators. Followers of these pages will not be surprised because they’ve contained many pieces describing the emerging complexity of both tumours and adjacent cells and tissues. We’ve seen that the mutation load rises as tumours go from early stages to metastatic growths (What’s New in Breast Cancers?, Family Tree of Breast Cancer). We’ve looked at snapshots of tissues that reveal the staggering complexity in terms of different types of cell coming and going in the tumour locale (the tumour microenvironment, TME in On The Slippery Slopes of Tumours). We’ve also seen how the tumour immune landscape can be perturbed in obesity by a metabolic tug of war between tumour and immune system cells (T cells) for circulating fats (Still Time for a Resolution).
Most arresting are the astonishing results of Keren et al. (Mosaic Masterpieces) who showed that in a large number of tissue sections of triple-negative breast cancers it was possible to pick out 36 different cell types — and that no two tumour slices showed the same make-up. Despite this bewildering variation they did manage to tease out some trends that related cell types in the TME to patient survival.
If at first …
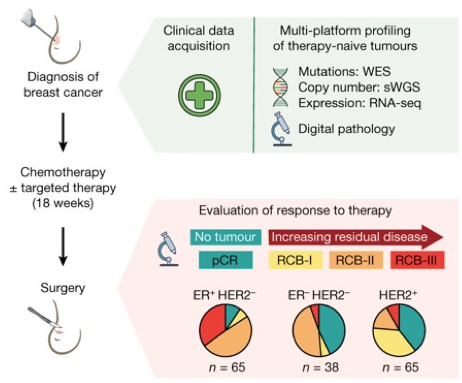
Stephen-John Sammut, Carlos Caldas and colleagues from the Cancer Research UK Cambridge Institute have taken a further step by pulling together as much data as they could gather from 168 patient samples (this included DNA sequencing to reveal mutational features and profiling the patterns of immune cells, e.g., T lymphocytes). These patients were then either treated with chemotherapy against HER2 or not before surgery.
Overview of the study design. Pre-therapy breast tumours from 168 patients were profiled using DNA sequencing and RNA sequencing (RNA-seq) and digital pathology analysis. These data were integrated within machine learning models to predict responses. Responses were assessed using the Residual Cancer Burden classification — the amount of residual disease after neoadjuvant chemotherapy. sWGS = shallow whole-genome sequencing; WES = whole-exome sequencing. pCR = pathological complete response. RCB-I, II and III refer to increasing levels of residual disease. From Sammut et al., 2022.
The upshot of the mass of data is that the extent of residual disease after therapy was associated with pre-therapy features, including tumour mutational and copy number landscapes, tumour proliferation, immune infiltration and T cell dysfunction — notably exclusion from the TME. Thus higher immune cell infiltration (raised numbers of CD8 T cells) was associated with a complete response.
In addition, in HER-ve tumours the rate of proliferation is a key determinant of response to chemotherapy — usually associated with high mutation load and chromosome instability, as well as specific mutation in, e.g., TP53 and BRCA genes.
Unexpectedly, in HER2+ tumours treated with chemotherapy and HER2-targeted antibodies (Trastuzumab/Herceptin), responses appeared to be independent of proliferation. This is presumably connected with the fact that the most dangerous feature of cancer cells is when they become able to metastasize—migrate from their original sites and establish new tumours in other parts of the body. However for cells to become invasive they suspend cell division.
And the message
This work doesn’t solve the problem of uncertainty when treating tumours with drugs, either before surgery or more generally. But its considerable success in dissecting important factors that determine the outcome for a set of breast cancers is a major pointer to a future in which ineffective use of drugs can progressively be minimised.
Reference
Sammut, SJ., Crispin-Ortuzar, M., Chin, SF. et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature 601, 623–629 (2022). https://doi.org/10.1038/s41586-021-04278-5

Pingback: Now for the Not Such Good News | Betrayed by Nature: The War on Cancer