I suspect that, of all the genes and proteins that have appeared over the years in these pages, MYC tops the frequency list (Taking the MYC out of Cancer, No Nodding Off at the Back, Two Heads Better Than One, A Moving Picture). The other two populists are almost certainly RAS and P53. It’s easy to see why MYC is such a favourite in cancer world. There are three genes in the MYC family (MYC, MYCN, MYCL) and they are deregulated (i.e. they make abnormal amounts of their proteins) in at least 50% of human cancers — 100% according to many pundits. MYC oncoproteins belong to a family of ‘super-transcription factors’, reckoned to have the potential to regulate the transcription of at least 15% of the entire genome.
Mighty MYC
Myc was discovered in 1982, following a brilliant piece of reasoning by Michael Bishop, as an oncogene in the avian myelocytomatosis virus MC29. Since then its human counterpart, MYC, has been shown to have a central role in almost every aspect of oncogenesis, dramatically summarised in the figure below from a review by Hui Chen and colleagues.

Cell activities regulated by MYC. MYC has been shown to bind to well over 3,000 genes out of 25,000 in the human genome, so perhaps the power of MYC as a gene expression and cell phenotype controller is not unexpected. From Chen et al. 2018.
Wood and trees
Despite the vast amounts of data implied in the above picture, a clear answer to the question “How does MYC drive cancer?” is tough to come up with. We know the presence of MYC protein is essential for cells to proliferate — the key feature of cancer. And proliferation needs energy — more proteins and suchlike so that cells can grow and reproduce. But how does that work?
A recent contribution from Peter Kreuzaler, Mariia Yuneva and friends from The Francis Crick Institute, London and other centres focussed on a specific aspect of MYC: cells with high levels of MYC increase their uptake of vitamin B5 (aka pantothenic acid), a nutrient that supports key metabolic processes. How do they do that?
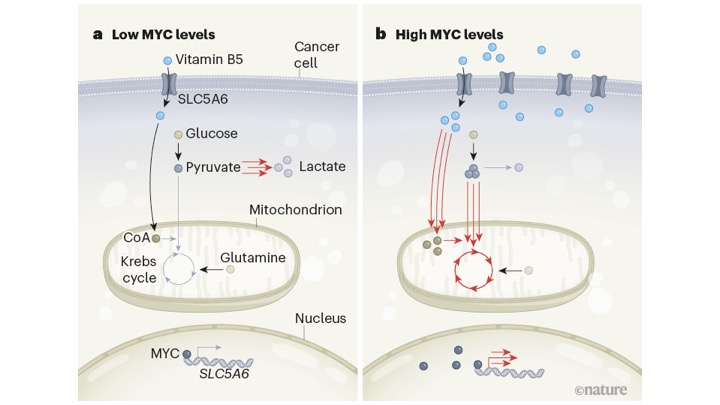
High levels of MYC protein associates with high levels of vitamin B5. From Kreuzaler et. 2023; scheme from Wallace 2023.
Kreuzaler and colleagues built a mouse model of triple-negative breast cancer in which tumours were driven by WNT1 (a secreted glycoprotein that activates various signal pathways that, uncontrolled, can drive cancers). The model also included a construct (MYC-ER) that can be switched on to give high levels of active MYC protein (by injecting the drug tamoxifen).
In a paper full of hugely challenging technical experiments they were able to compare the metabolic changes in cells with low and high levels of MYC. The key finding was that tumours expressing high levels of MYC also produced a lot of the protein SLC5A6, which can transport vitamin B5 into cells. SLC5A6 was only weakly expressed in low MYC tumours.
The above scheme shows the idea that when the local levels of MYC go up they affect control regions of the gene encoding SLC5A6. Result: more SLC5A6 — and more vitamin B5 in cells. Vitamin B5 attaches to CoA to play a key role in getting carbohydrates (glucose) and fatty acids into the Krebs cycle.
Overall then, in cells with low MYC levels (not many vitamin B5 transporters) more molecules of glucose and pyruvate are routed to make lactate than feed into the Krebs cycle (left hand figure red arrows). Conversely, in cells with high levels of MYC, high expression of SLC5A6 promotes uptake of vitamin B5. More molecules enter the Krebs cycle than give rise to lactate (right hand figure red arrows).
Why is it important to know this level of detail?
The huge spectrum of MYC gene targets suggests that the obvious approach to therapy of blocking MYC might kill cancers but in the process might also kill the host — which is why for decades MYC has been said to be ‘undruggable’: its direct targeting would probably be fatal.
However, the action of abnormally raised MYC on energy supply for the cell (by driving vitamin B5 uptake) may offer a specific target, blockade of which would restrict the growth of tumour cells without effect on the rest of the organism.
Those familiar with the long-running MYC saga will not be holding their breaths but even if this work does not lead to a new therapy it still provides a remarkable insight into the workings of the this enigmatic and immensely powerful little regulator.
References
Chen, H., Liu, H. & Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Sig Transduct Target Ther 3, 5 (2018). https://doi.org/10.1038/s41392-018-0008-7
Kreuzaler, P., Inglese, P., Ghanate, A. et al. Vitamin B5 supports MYC oncogenic metabolism and tumor progression in breast cancer. Nat Metab 5, 1870–1886 (2023). https://doi.org/10.1038/s42255-023-00915-7
Wallace M. MYC protein helps cancer to take its vitamins. Nature. 2023 Dec;624(7991):258-260. doi: 10.1038/d41586-023-03764-2. PMID: 38086940.
