In the second type of cancer immunotherapy a sample of a patient’s T lymphocytes is grown in the lab. This permits either expansion of the number of cells that recognize the tumour or genetic engineering to modify the cells so they express receptors on their surface that target them to the tumour cell surface. Infusion of these manipulated cells into the patient enhances tumour cell killing. We’re now in the realms of ‘personalized medicine’.
A little more of a good thing
The first of these methods picks up a weakness in the patient’s immune system whereby it makes lymphocytes that kill tumour cells but can’t make enough – their protective effect is overwhelmed by the growing cancer. By taking small pieces of surgically removed tumours and growing them in the lab, it’s possible to select those T cells that have killing capacity. These are expanded over a few weeks to make enough cells to keep on growing when they’re infused back into the patient. The upshot is a hefty boost for the natural anti-tumour defence system. The pioneer of this method, called adoptive cell therapy, is Steven Rosenberg (National Cancer Institute, Bethesda) and it has been particularly effective for melanomas. Responses are substantially improved by treatment with drugs that reduce the white cell count before samples are taken for T cell selection – probably because the system responds by making growth factors to restore the balance and these drive the expansion of the infused cells.
A wonderful benefit of this method is its efficacy against metastases – i.e. tumour growths that have spread from the primary site – perhaps not surprising as it’s what Rosenberg calls a “living” treatment, in other words it just gives a helping hand to what nature is already trying to do.
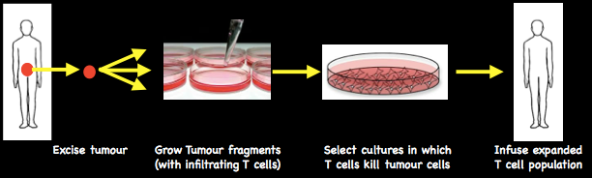 Selecting naturally occurring T cells with anti-tumour activity
Selecting naturally occurring T cells with anti-tumour activity
Tumour fragments are grown in the laboratory: lymphocytes that kill tumour cells are selected and expanded in culture. About 6 weeks growth yields enough cells to infuse into the patient.
Gene therapy
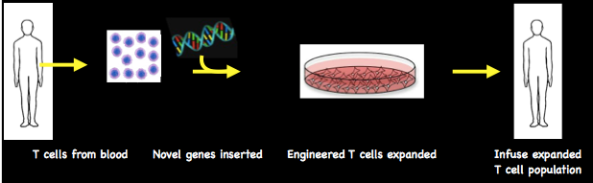
A more sophisticated approach to boosting innate immunity is to introduce new genes into the genetic material (the genome) of T cells to target them to tumour cells with greater efficiency. An ordinary blood sample suffices as a starting point from which T cells are isolated. One way of getting them to take up novel genes uses viruses – essentially just genetic material wrapped in an envelope. The virus is ‘disabled’ so that it has none of its original disease-causing capacity but retains infectivity – it sticks to cells. ‘Disabling’ means taking just enough of the original genome to make the virus – a viral skeleton – and then inserting your favourite gene, so the engineered form is just a handy vehicle for carrying genes. No need to panic, therefore, if you see a press headline of the “HIV cures cancer” variety: it just means that the human immunodeficiency virus – well and truly disabled – has been used as the gene carrier.
Genetic modification of blood lymphocytes
T cells are isolated from a blood sample and novel genes inserted into their DNA. The engineered T cells are expanded and then infused into the patient.
This method of re-directing T cells to a desired target was pioneered by Gideon Gross and colleagues at The Weizmann Institute of Science in Israel in the late 1980s and it has led to sensational recent results in treating chronic lymphocytic leukemia (CLL), albeit in just a few patients so far. To the fore have been Renier Brentjens and his group from the Memorial Sloan-Kettering Cancer Center, New York. The genetic modification they used made the patient’s T cells express an artificial receptor on their surface (called a chimeric antigen receptor). This T cell receptor was designed to stick specifically to a protein known to be displayed on the surface of CLL cells. The result was that the T cells, originally unable to ‘see’ the leukemic cells, now homed in on them with high efficiency. Astonishingly, and wonderfully, the modified cells divide in the patient so that, in effect, their immune system has been permanently super-charged.
A critical part of the strategy is that CLL cells carry a known molecular target but the absence of such defined markers for most cancers is currently a severe limitation. On the bright side, however, this type of gene therapy has now been attempted in at least three different centres and, despite inevitable minor differences in method, it clearly works.
One of the leading figures in gene therapy is Carl June of the University of Pennsylvania. Some of his colleagues have made a brilliant video explaining how it works whilst June himself has described in wonderfully humble fashion what it means to work in this field.
References
Rosenberg, S.A. and Restifo, N.P. (2015). Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62-68.
Gross, G., et al. (1989). Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptorswith antibody-type specificity. Proc. Natl. Acad. Sci. U.S.A. 86, 10024–10028.
Brentjens, R.J., et al. (2013). CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med., 5, 177ra38. DOI:10.1126/scitranslmed.3005930.
Kalos, M., et al. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73.
Kochenderfer, J.N., et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 119, 2709–2720.