Over a century ago there lived in London an astute physician by the name of Stephen Paget. He was one of those who may or may not be envied in being part of a super-talented family. His Dad, Sir James Paget, was pals with Charles Darwin and, together with Rudolph Virchow, laid the foundations of modern pathology, though today medical students usually encounter his infinitesimal immortality through several diseases that bear his name. These include a rare condition, Paget’s disease of the breast, in which malignant cells form in the skin of the nipple creating an itchy rash, usually treatable by surgery. His Uncle George had been Regius Professor of Physic at Cambridge and he had several brothers, two of whom became bishops. Fortunately Stephen continued the medical thread of the family and Paget’s passion became breast cancer.
A Key Question
Paget had that invaluable scientific gift of being able to pinpoint a key question – in his case ‘What is it that allows tumour cells to spread around the body?’ – and it was such a good question that to this day we don’t have a complete answer. That it happens had been known long before the appearance of Paget Junior. René-Théophile-Hyacinthe Laënnec, French of course, in the early years of the 19th century described how skin cancer could spread to the lungs before he went on to invent the stethoscope in 1816. The mother of this invention was a young lady whom he described as having a ‘great degree of fatness’ that made her heartbeat inaudible by the then conventional method of placing ear to chest. Using a piece of paper rolled into a tube as a bridge, Laënnec was somewhat taken aback that the beat was more distinct than he’d ever heard before. Needless to say, medicine being a somewhat reactionary profession, not all its practitioners had ears tuned to receive this advance with glee but in the end, of course, it caught on and we can therefore award Laënnec first prize in reducing human cumulative embarrassment. It was another French surgeon, Joseph Récamier, who subsequently coined the term metastasis, (to be precise ‘métastase’) to describe the formation of secondary growths derived from a primary tumour.
Early Ideas about Metastasis
The notion that primary tumours could give rise to a diaspora gradually took root but it was not until 1840 that the Munich-born surgeon Karl Thiersch showed that it was actually cells – malignant cells – that wandered off and found new homes. Rudolf Virchow had come up with the idea that spreading was via a ‘juice’ released by primaries that somehow converted normal cells at other sites into tumours. As Virchow was jolly famous, having not only made the study of disease into a science but also discovered leukemia, it took a while for Thiersch to triumph, notwithstanding the evidence of Laënnec and others. Funnily enough, and as quite often happens in scientific arguments, it now looks as though both were right if for ‘juice’ you substitute ‘messengers’ – that is, chemicals dispatched by tumour cells – as we shall see.
Paget’s attention had been drawn to this subject through his observations on breast cancer, and he’d taking as a starting point the most obvious question: ‘How do tumour cells know where to stick?’ Or, as he elegantly phrased it in a landmark paper of 1889: ‘What is it that decides what organs shall suffer in a case of disseminated cancer?’ The simplest answer would be that it just depends on anatomy: when cells leave a tumour and get into the circulation they stick to the first tissue they meet. But in looking at over 700 cases he’d found this just didn’t happen and that secondary growths often appeared in the lungs, kidneys, spleen and bone. Paget acknowledged the uncommonly prescient suggestion a few years earlier by Ernst Fuchs that certain organs may be ‘predisposed’ for secondary cancer and concluded that ‘the distribution of secondary growths was not a matter of chance.’ This led him to a botanical analogy for tumour metastasis: ‘When a plant goes to seed, its seeds are carried in all directions; but they can only live and grow if they fall on congenial soil.’ From this, then, emerged the ‘seed and soil’ theory of metastasis, its great strength being the image of interplay between tumour cells and normal cells, their actions collectively determining the outcome. Rather charmingly, Paget concluded his paper with: ‘The best work in the pathology of cancer is now done by those who are studying the nature of the seed. They are like scientific botanists; and he who turns over the records of cases of cancer is only a ploughman, but his observation of the properties of the soil may also be useful.’

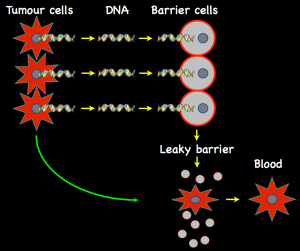
Bookmarking cancer: Primary tumours mark sites around the body to which they will spread (metastasize) by sending out chemical signals that create sticky ‘landing sites’ (red protein A) on target cells. Cells released from the bone marrow carry proteins B and C. B attaches to A and tumour cells ‘land’ on C. Cells may remain quiescent in a new site for years or decades, their growth suppressed by signals (e.g., TSP-1) released from nearby blood vessels. Only when appropriate activating signals dominate (e.g., TGFbeta) is secondary tumour growth switched on.
Finding a Landing Strip
For well over a century Paget’s aphorism of ‘seed and soil’ pretty well summed up our knowledge of metastasis. It’s obvious that before any rational therapy can be designed we need to unravel the molecular detail but we’ve had to wait until the twenty-first century for any further significant insight into the process. As so often in science, the hold-up has been largely due to waiting for the appropriate combination of methods to be developed – in this case fluorescently tagged antibodies to detect specific proteins in cells and tissues and genetically modified mice.
In the forefront of this pursuit has been David Lyden and his colleagues at Weill Cornell Medical College and other centers and their most extraordinary finding is that cells in the primary tumour release proteins into the circulation and these, in effect, tag what will become landing points for wandering cells. Extraordinary because it means that these sites are determined before any tumour cells actually set foot outside the confines of the primary tumour. These are chemical messengers rather equivalent to Virchow’s ‘juice’: they don’t change normal cells into tumour cells but they do direct operations. However, it’s a bit more complicated because, in addition to sending out a target marker, tumours also release proteins that signal to the bone marrow. This is the place where the cells that circulate in our bodies (red cells, white cells, etc.) are made from stem cells. The arrival of signals from the tumour causes some cells to be released into the circulation; these carry two protein markers on their surface: one sticks to the pre-marked landing site, the other to tumour cells once they appear in the circulation. It’s a double-tagging process: the first messenger makes a sticky patch for bone marrow cells that appear courtesy of another messenger, and they become the tumour cell target. It’s molecular Velcro: David Lyden calls it ‘cellular bookmarking.’
Controlling Metastatic Takeoff
Tumour cells that find a new home in this way, after they’ve burrowed out of the circulation, could in principle then take off, growing and expanding as a ‘secondary.’ However, and perhaps surprisingly, generally they do the exact opposite: they go into a state of hibernation, remaining dormant for months or years until some trigger finally sets them off. The same group has now modeled this ‘pre-metastatic niche’ for human breast cancer cells, showing that the switch between dormancy and take-off is controlled by proteins released by nearby blood vessels. The critical protein that locks tumour cells into hibernation appears to be TSP-1 (thrombospondin-1). As long as TSP-1 is made by the blood vessel cells metastatic growth is suppressed. This effect is overridden by stimuli that turn on new vessel growth and in so doing switch secretion from TSP-1 to TGFB (transforming growth factor beta). Now proliferation of the disseminated tumour cells is activated and the micro-metastasis becomes fully malignant. It should be said that this is a model system and may possibly bear little relation to what goes on in real tumours. However, the fact that specific proteins that are, moreover, highly plausible candidates, can control such a switch strongly suggests its relevance and also highlights potential targets for therapeutic manipulation.
Stranger Than Fiction
The system for directing tumour cells to a target seems extraordinarily elaborate. Given that tumour cells cannot evolve in the sense of getting better at being metastatic – they just have to go with what they’ve got – how on earth might it have come about? We don’t know, but the most likely explanation is that they are taking advantage of natural defense mechanisms. Although tumours start from normal cells, the first reaction of the body is to see them as ‘foreign’ – much as it does bugs that get into a cut – and the response is to switch on inflammation and an immune response to eliminate the ‘invader.’
Perhaps what is happening in these mouse models is that the proteins released by the tumour cells are just a by-product of the genetic disruption in cancer cells. Nevertheless, they may signal ‘damage somewhere in the body’. That at least would explain why the bone marrow decides to release cells that are, in effect, a response to the tumour. The second question is trickier: Why should tumours release proteins that mark specific sites? We’ve known since Dr. P’s studies that cells from different tumours do indeed head for different places and it may just be that the messengers arising in the genetic mayhem happen to reflect the tissue of origin. The mouse models, encouragingly, show that the target changes with tumour type (e.g., swap from breast to skin and the cells go somewhere else). In other words, tumours send out their own protein messengers that set up sticky landing strips in different places around the circulation.
As for take-off, it may be that newly arrived tumour cells simply adapt to the style of their neighborhood. By and large, the blood vessels are pretty static structures: they don’t go in for cell proliferation unless told to do so by specific signals, as happens when you get injured and need to repair the damage. TSP-1 appears to be a ‘quiescence’ signal, telling cells to sit tight. The switch to proliferation comes when that signal is overcome by TGFB, activating both blood vessels and tumour. All of which would delight Paget: not only is our expanding picture consistent with ‘seed and soil’ but the control by local signals over what happens next makes his rider that ‘observation of the properties of the soil may also be useful’ spot on.
References
Kaplan, R.N., Riba, R.D., Zacharoulis, S., Bramley, A.H., Vincent, L., Costa, C., MacDonald, D.D., Jin, D.K., Shido, K., Kerns, S.A., Zhu, Z., Hicklin, D., Wu, Y., Port, J.L., Altork, N., Port, E.R., Ruggero, D., Shmelkov, S.V., Jensen, K.K., Rafii, S. and Lyden, D. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820-827.
http://www.ncbi.nlm.nih.gov/pubmed/16341007
Ghajar, C.M. et al. (2013). The perivascular niche regulates breast tumour dormancy. Nature Cell Biology 15, 807–817.
http://www.readcube.com/articles/10.1038/ncb2767